Client: Peter MacCallum Cancer Centre
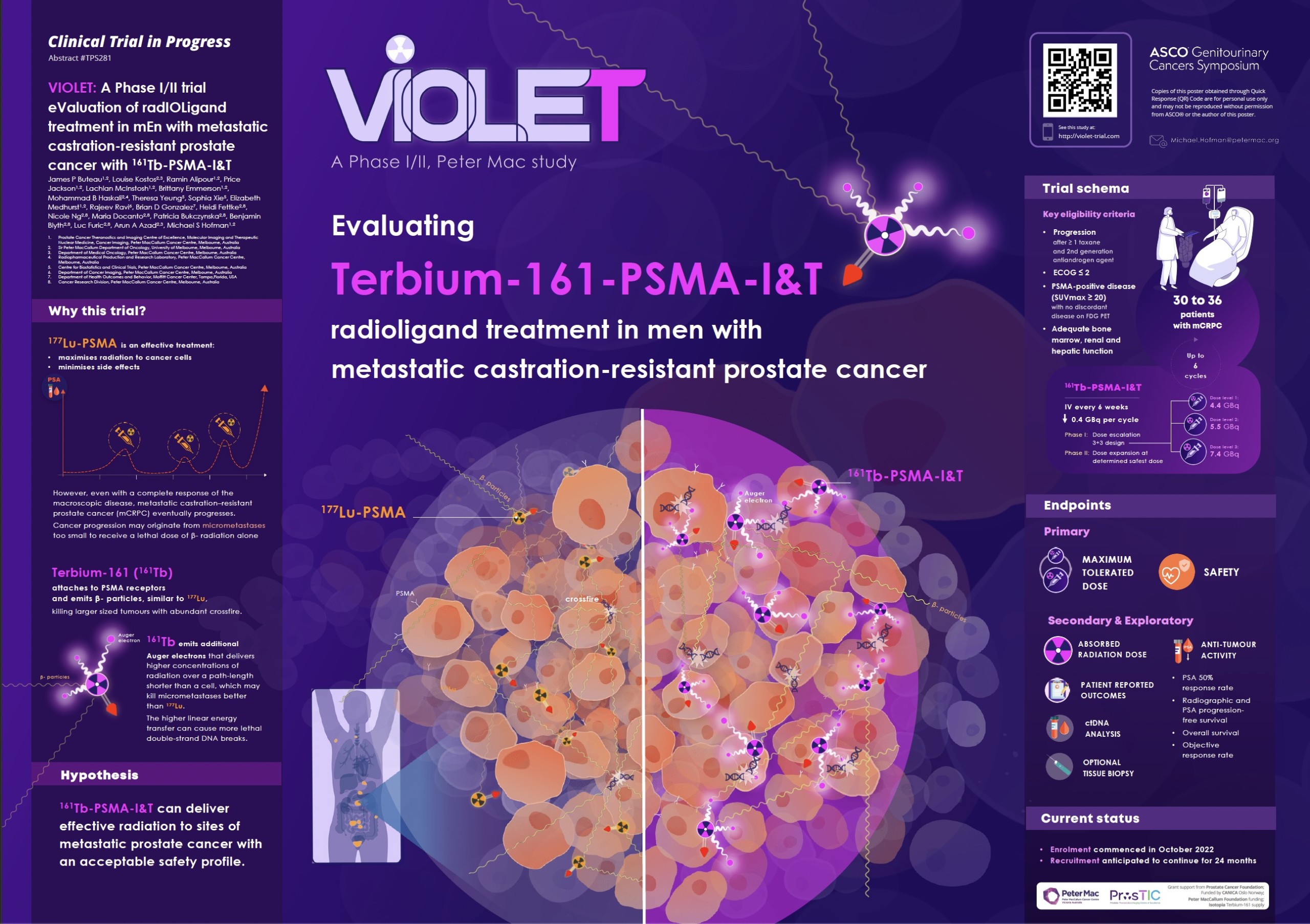
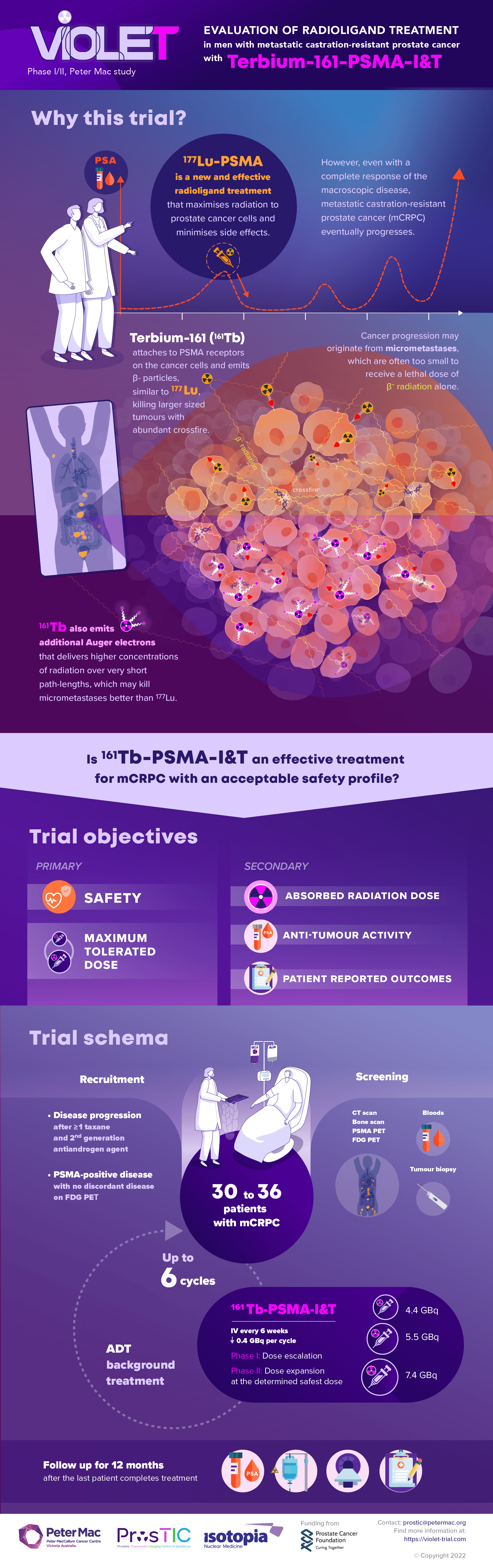
Context: VIOLET: EValuation of radIOLigand treatment in mEn with metastatic castration-resistant prostate cancer with [161Tb]Tb-PSMA-I&T: phase I/II study
This prospective, single-centre, single-arm phase I/II trial aiming to evaluate the safety and effectiveness treatment with Terbium 161 PSMA I&T.
PSMA theranostics is a treatment increasingly being used for people with advanced prostate cancer and the focus of the world-leading research at ProsTIC at Peter Mac. This therapy aims to reduce the size of tumours and to stop them from multiplying, as well as to ease the symptoms that people may get.
Phase I of the trial aims to establish the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and recommended phase 2 dose (RP2D) of [161Tb]Tb -PSMA-I&T in patients with metastatic castration-resistant prostate cancer (mCRPC).
Phase II aims to evaluate the safety of [161Tb]Tb -PSMA-I&T in patients with mCRPC.
Read more information on clinicaltrials.gov: NCT05521412
What we did:
- Long form explainer infographic of the Research trial
- Animated Powerpoint presentation based on the infographic
- ASCO GU conference scientific poster
ASCO GU conference 2023 – Research poster
designed with the #betterposter approach in mind.
(Watch Mike Morrison #betterposter reasoning here)