Client: Peter McCallum Cancer Centre
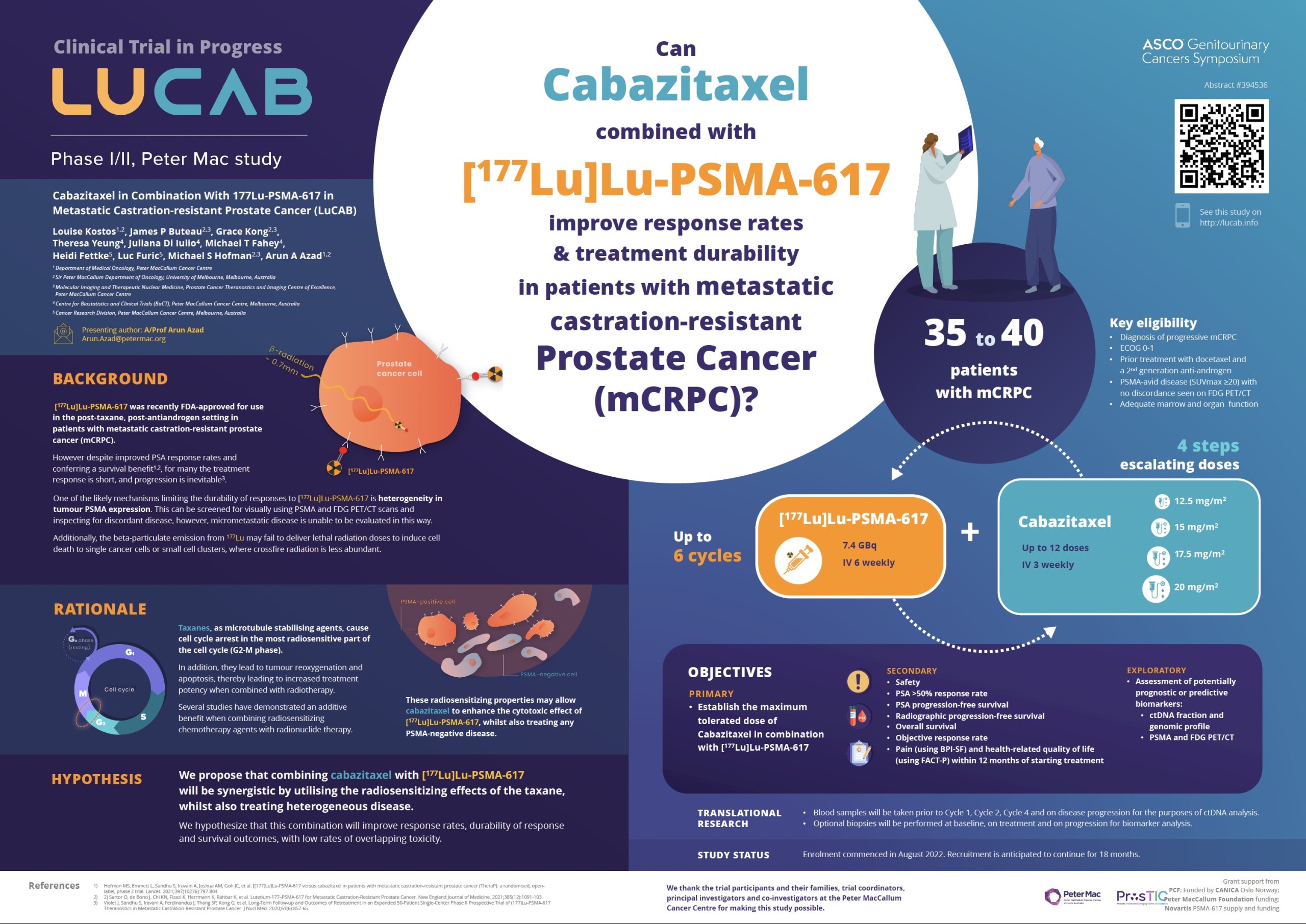
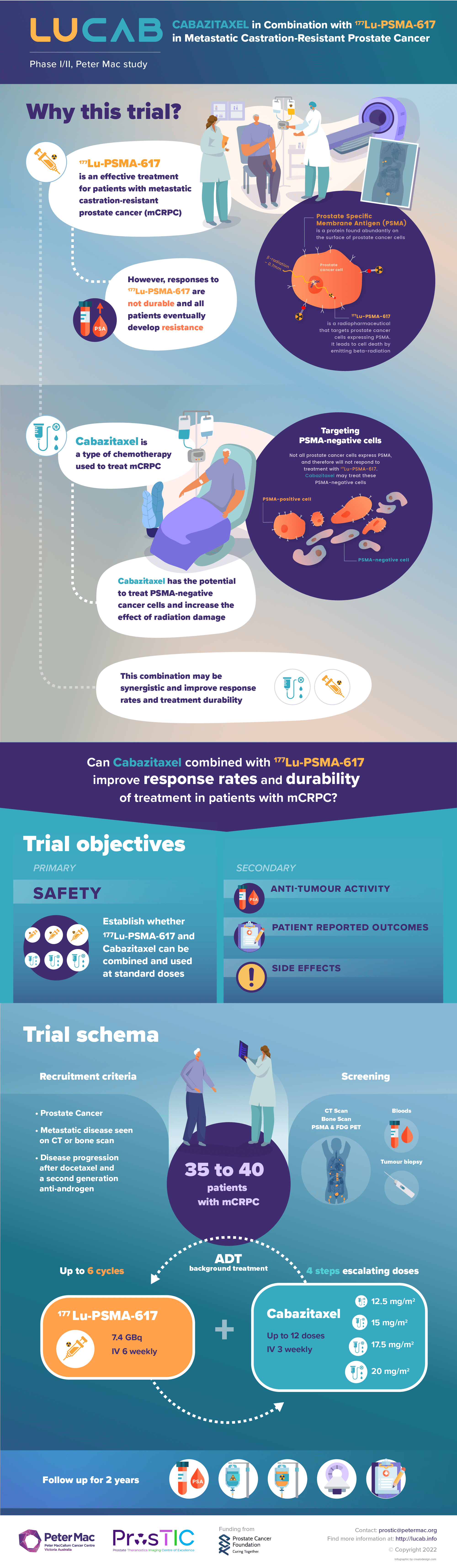
Context: Theranostic treatment for prostate cancer involves targeting prostate specific membrane antigen (PSMA) on prostate cancer cells – first with a radioactive molecule to reveal the cancer’s spread via a PET scan and then a similar radioactive molecule that kills cancer cells. Peter Mac has pioneered research globally in this field. LuCAB is a prospective phase I/II trial to establish the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and recommended phase 2 dose (RP2D) of Cabazitaxel in combination with 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer (mCRPC).
This infographic, supported by an animated Powerpoint presentation, provides a snapshot understanding of the trial’s context, reasoning and protocol. It is aimed at both the medical research community and potential patients.